In Vitro Comparative Study of the Survival of Probiotic Capsules in a Simulated Gastric Environment
Abstract
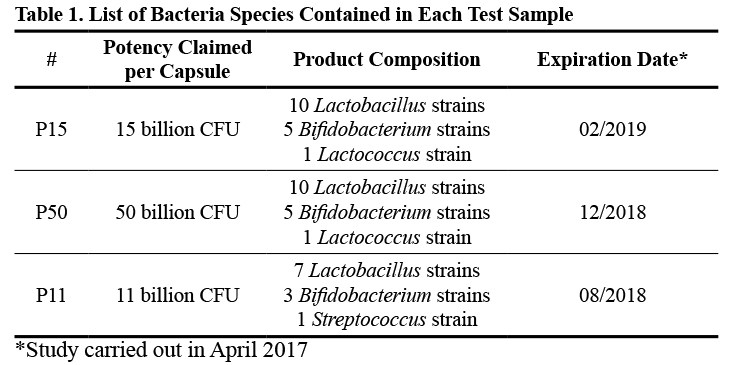
Three commercial probiotic products containing primarily Lactobacillus and Bifidobacterium species were investigated. They were identified as P15, P50, and P11. All three products are encapsulated in vegetable-sourced capsules, with P11 being enteric-coated. The objective of this study was to evaluate capsule disintegration and microbial survival of probiotic strains upon exposure to simulated gastric conditions. Capsule disintegration was performed respecting the protocol of the United States Pharmacopoeia (USP).
Probiotic samples were incubated for defined periods of time in a simulated gastric environment, then neutralized and plated on a suitable culture media. After 60 minutes,
the results showed that enteric-coated probiotic capsules ensure that microorganisms better survive destructive gastric acidic conditions.
Introduction
Probiotics of the genera Lactobacillus and Bifidobacterium have the potential to provide therapeutic and health benefits to humans (Kailasapathy and Chin, 2000). Benefits include (i) immune response enhancement, (ii) prevention of diarrheal diseases, (iii) prevention of hypercholesterolemia, (iv) improvement in lactose utilization, (v) prevention of upper gastrointestinal tract diseases, and (vi) stabilization of the gut mucosal barrier (Kailasapathy and Chin, 2000). To maximize these benefits, probiotics must resist degradation by digestive enzymes, be resistant to the action of bile salts, and survive the gastric acidic conditions (Kailasapathy and Chin, 2000). The supplement industry employs various types of capsules with or without enteric coating. The objective of this study was to evaluate capsule disintegration and microbial survival of probiotic strains upon exposure to simulated gastric fluid (SGF).
Materials and Methodology
Test Samples and Microorganisms
Three commercial probiotic products were selected for investigation. The products are summarized below.
Simulation of the Gastric Conditions and Disintegration Testing
Gastric conditions were simulated by preparing simulated gastric fluid (SGF). The pH of the solution was 1.2. Capsules were randomly selected then incubated in the SGF for 30 min and 60 min. Disintegration test was conducted in compliance with USP protocol.
Results and Discussion
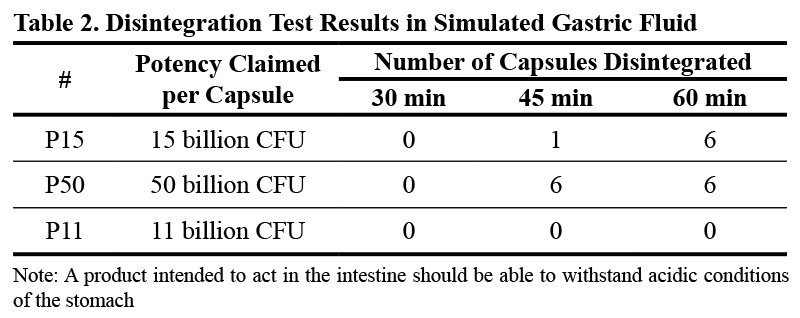
Disintegration Test
Up to 30 min, all capsules irrespective of the manufacturer withstood acidic conditions. P15 started to disintegrate at 45 min with one capsule, and completely disintegrated at 60 min. As for P50, all six capsules disintegrated at 45 min. All capsules of P11 resisted disintegration at the end of the 60-minute stay in SGF. This could be due to the enteric coating, which acts by protecting its contents prior to reaching the small intestine.
Results presented in Table 2 suggest that products P15 and P50 may be exposed to acidic conditions that affect the viability of the probiotics they contain. Studies have reported that the average time of exposure of food including drugs to the action of gastric fluid is more than 60 minutes (Camilleri et al, 1989).
Resistance to Artificial Gastric Fluid
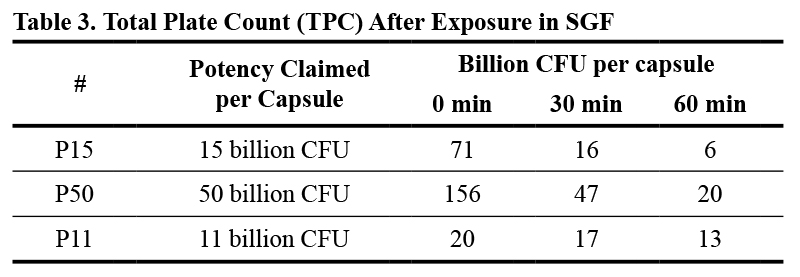
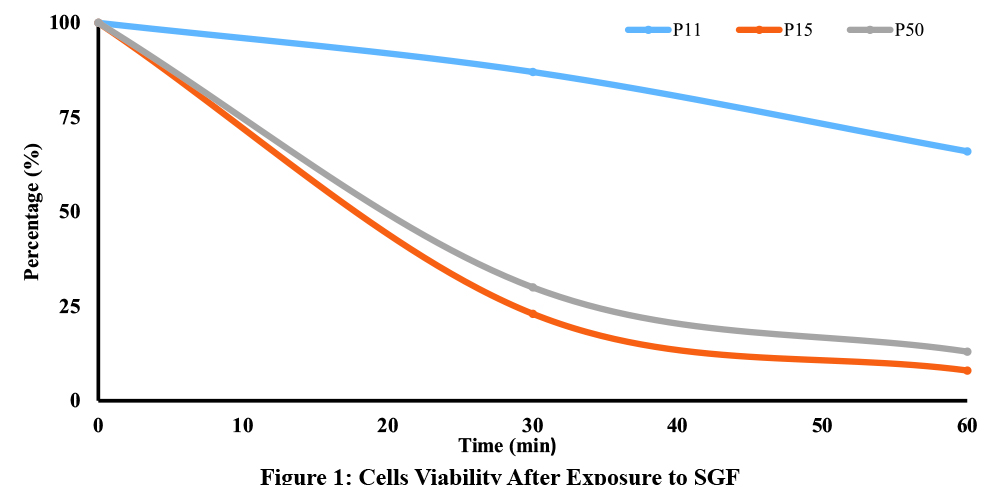
The effect of exposure to simulated gastric fluid was tested at 30 min and 60 min. Results of this comparative investigation are shown in Table 3. On the other hand, Figure 1 shows the percentage of reduction of total plate count (TPC) relative to the initial count of probiotics in the three tested products.
This illustration provides evidence of the impact of acidic conditions on probiotics. P15 and P50 exhibited 8% and 13% viable cells, respectively, versus 66% for P11 (Fig. 1) following 60 minutes of incubation. After 30 min, this viability was calculated to be 23%, 30%, and 87 % for P15, P50, and P11, respectively. These results show that Lactobacillus and Bifidobacterium species are sensitive to acidic conditions. These bacteria inhabit human intestines where the pH is neutral, between 6 and 7.5 (Maurer, J.M. et al, 2015). This demonstrates the critical importance of enteric coating for delayed release dosage.
The high loss rate observed in P15 and P50 might account for the considerable overbuilding during the production (473% and 312% for P15 and P50, respectively). Nevertheless, this overbuilding appears insufficient, as only about 40% of the claimed cells remained viable after 60 min in the simulated gastric fluid. In contrast, P11, with a more modest overbuild of 182%, still exceeded the claim, with 118% survival of viable cells.
Conclusions
Gastric acidic conditions are harsh and detrimental to most probiotic strains. As demonstrated in this study, survival of probiotic bacteria in acidic conditions is highly contingent on capsules surviving harsh gastric conditions. Non–enteric-coated capsules demonstrated poor efficacy, whereas enteric-coated capsules demonstrated higher viability. This was determined by both disintegration data and enumeration data following exposure to SGF.
References
Camilleri, M., et al. “Human gastric emptying and colonic filling of solids characterized by a new method.” American Journal of Physiology-Gastrointestinal and Liver Physiology. Vol. 257, No. 2 (1989): G284–G290.
Kailasapathy, K., and J. Chin. “Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp.” Immunology and Cell Biology. Vol. 78, No. 1 (2000): 80–88.
Maurer, J.M. et al. “Gastrointestinal pH and Transit Time Profiling in Healthy Volunteers Using the IntelliCap System Confirms Ileo-Colonic Release of ColoPulse Tablets.” PLoS ONE Vol. 10, No. 7 (2015): e0129076.

 Stores
Stores